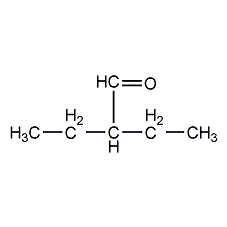
Structural formula
| Business number | 02CY |
|---|---|
| Molecular formula | C6H12O |
| Molecular weight | 100.16 |
| label |
3-formylpentane, a-ethylbutyraldehyde, 3-Formylpentane, 2-Ethylbutanal |
Numbering system
CAS number:97-96-1
MDL number:MFCD00006985
EINECS number:202-623-5
RTECS number:ES2625000
BRN number:1209330
PubChem number:24846958
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25℃): 0.81-0.84
3. Relative vapor density (g/mL, air=1): 3.45
4. Melting point (ºC): -89
5. Boiling point (ºC, normal pressure): 117~119 (21.3kpa)
6. Relative density (20℃, 4℃): 0.8150
7. Refractive index (n20D): 1.402
8. Flash point (ºC): 21.1
9. Relative density (25℃, 4℃): 0.8106
10. Refractive index at room temperature (n20): 1.4024
11. Refractive index at room temperature (n25): 1.4002
12. Saturated vapor pressure (kPa, 20ºC): 1.83
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): 7.7
18. Lower explosion limit (%, V/V): 1.2
19. Solubility: Insoluble in water, miscible in alcohol and ether.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 500mgREACTION SEVERITY, slight reaction; 2. Acute toxicity: rat oral LD50: 3980mg/kg; rat inhalation LCLo: 8000ppm/4H; rabbit skin contact LD50 :5990μL/kg;
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 29.99
2. Molar volume (cm3/mol): 125.2
3. Isotonic specific volume (90.2K ): 277.1
4. Surface tension (dyne/cm): 23.9
5. Polarizability (10-24cm3): 11.88
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.5
2. Number of hydrogen bond donors: 0
3.�Number of � bond receptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecules Polar surface area 17.1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 46.1
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong alkali, strong reducing agents and air.
2. Found in flue-cured tobacco leaves.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 10℃. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, reducing agents, alkalis, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. It is formed by the condensation of diethylmethanol and anhydrous oxalic acid or sulfuric acid.
2. The new method is obtained by reacting a-Z, alkenyl crotonal with iron filings and acetic acid.
3. Tobacco: FC, 40.
Purpose
Used in organic synthesis, mixed with triethylaluminum and used as a two-component igniter in rocket propulsion systems.
extended-reading:https://www.newtopchem.com/archives/44272extended-reading:https://www.cyclohexylamine.net/dabco-amine-catalyst-soft-foam-catalyst-dabco/extended-reading:https://www.bdmaee.net/cyclohexylamine-series-products-2/extended-reading:https://www.bdmaee.net/niax-a-1/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/40.jpgextended-reading:https://www.cyclohexylamine.net/n-dimethylaminopropyldiisopropanolamine-cas-63469-23-8/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/52.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/25.jpgextended-reading:https://www.newtopchem.com/archives/44097extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/drier-butyl-tin-oxide-FASCAT-4101.pdf
