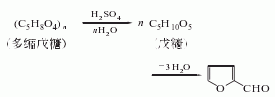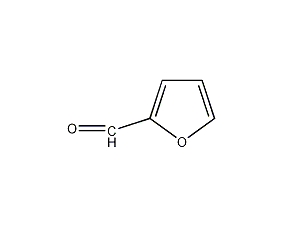
Structural formula
| Business number | 02D2 |
|---|---|
| Molecular formula | C5H4O2 |
| Molecular weight | 96.09 |
| label |
2-Furancarbaldehyde, α-furancarbaldehyde, Gluten creates ant oil, Pyroviscosity aldehyde, Furan formaldehyde, 2-Formylofuran, 2-Furancarboxaldhyde, Furfurol, Pyromucic aldehyde, Furfural, Extracting agent, Multifunctional solvents, heterocyclic compounds, organic insulating materials, synthetic raw materials, Intermediates |
Numbering system
CAS number:98-01-1
MDL number:MFCD00003229
EINECS number:202-627-7
RTECS number:LT7000000
BRN number:105755
PubChem ID:None
Physical property data
1. Properties: colorless to yellow oily liquid with almond-like odor. [1]
2. Melting point (℃): -36.5[2]
3. Boiling point (℃): 161.8[3]
4. Relative density (water = 1): 1.16[4]
5. Relative vapor Density (air=1): 3.31[5]
6. Saturated vapor pressure (kPa): 0.27 (20℃)[6]
7. Heat of combustion (kJ/mol): -2338.7[7]
8. Critical pressure (MPa): 5.5[8]
9. Octanol/water partition coefficient: 0.41~0.69[9]
10. Flash point (℃): 60 ( CC)[10]
11. Ignition temperature (℃): 315[11]
12. Explosion limit (%): 19.3[12]
13. Lower explosion limit (%): 2.1[13]
14. Solubility: Slightly soluble in cold water, soluble in hot water, ethanol, ether, and benzene. [14]
15. Refractive index (20ºC): 1.52608
16. Refractive index (25ºC): 1.52345
17 .Ignition point (ºC): 490
18. Heat of evaporation (KJ/mol): 43.25
19. Heat of fusion (KJ/mol): 14.36
20. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.64
21. Solubility parameter (J·cm-3)0.5:23.644
22. van der Waals area (cm2·mol-1): 6.120×109
23. van der Waals volume (cm3·mol-1): 47.260
24. Gas phase standard Heat of combustion (enthalpy) (kJ·mol-1): -2388.2
25. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -151.0
26. Gas phase standard entropy (J·mol-1·K-1): 333.29
27. Gas phase standard free energy of formation (kJ·mol-1): -102.9
28. Liquid phase standard combustion heat (enthalpy) (kJ·mol– 1): -2337.6
29. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -201.6
30. Liquid phase standard entropy (J·mol-1·K-1): 217.99
31. Liquid phase standard formation free energy (kJ·mol– 1): -119.1
32. Liquid phase standard hot melt (J·mol-1·K-1): 169.0
Toxicological data
1. Acute toxicity: mouse oral LC50: 425 mg/kg; rat inhalation LD50: 601mg/m3, 4 hours; mouse abdominal LC50: 1490 mg/kg; dog oral LD50: 2300mg/kg; Guinea pig oral 541.7mg/kg;
2. Acute toxicity[15]
LD50: 65mg/kg (rat oral)
LC50: 175ppm (rat inhalation, 6h)
3. Irritation[16 ]
Rabbit transdermal: 500mg (24h), moderate irritation.
Rabbit eye: 20mg (24h), severe irritation.
4. Subacute and chronic toxicity[17] Dog inhalation 507mg/m3, 6 hours a day, 5 days a week, for a total of 4 weeks, resulting in hepatic steatosis.
5. Mutagenicity[18] Microbial mutagenicity: Salmonella typhimurium 8094μg/dish. Cytogenetic analysis: hamster ovary 2500 μmol/L. DNA inhibition: human HeLa cells 3mmol/L. Sister chromatid exchange: human lymphocytes 70 μmol/L. Unprogrammed DNA synthesis: human liver 2nmol/L (24h).
6. Carcinogenicity[19] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals .
Ecological data
1. Ecotoxicity[20]
LC50: 24~32mg/L (96h) (fish )
IC50: 2.7~31mg/L (72h) (algae)
2. Biodegradability[21] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, 93.5% degradation after 2 weeks.
3. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 25.30
2. Molar volume (cm3/mol): 83.8
3. Isotonic specific volume (90.2K ): 206.3
4. Surface tension (dyne/cm): 36.5
5. Polarizability: 10.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 30.2
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 70.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is non-corrosive to metals and can be stored in iron, mild steel, copper or aluminum containers. It gradually turns brown in the air or when exposed to light, so it should be protected from light and sealed with inert gas for storage. After furfural is placed in oxygen, air, carbon dioxide, and nitrogen for 40 days, the amounts of oxides (resin-like) generated are 1.7%, 0.3%, 0.1%, and 0.05% respectively. Adding p-hydroxydiphenylamine, diphenylamine, cadmium iodide, hydroquinone, pyrogallol or β-naphthol, etc. to furfural in an amount of 0.1% can effectively prevent oxidation. Adding 0.001% to 0.1% of N-phenyl substituted urea, thiourea or naphthylamine to furfural can prevent the formation of resin when heated at 60 to 170°C.
2. Chemical properties: Furfural has the properties of aldehydes. For example, it can form an addition compound with sodium bisulfite, which can be oxidized to furancarboxylic acid and reduced to furfuryl alcohol. Cannizzaro reaction occurs in concentrated potassium hydroxide solution to generate furfuryl alcohol and furancarboxylic acid. Reacts with potassium cyanide to form furfural (furoin). It reacts with ammonia to form furfuramide and reacts with amines to form Schiff base. Furfural and soda lime are heated together to 350~400°C, or nickel or zinc oxide and chromium or vanadium pentoxide are used as catalysts to heat to 200°C to convert into furan. When the refined furfural is placed, due to the action of oxygen in the air, a series of complex reactions such as decomposition and polymerization occur, making the color darker. Heating or light can accelerate its decomposition and polymerization reactions.
3. Stability[22] Stable
4. Incompatible substances[23] Strong oxidizing agent, strong alkali
5. Conditions to avoid contact[24] Heat, light, contact with air
6. Aggregation hazards[25] Aggregation
Storage method
Storage Precautions[26] Store in a cool, ventilated warehouse. The storage temperature should not exceed 37℃. Keep away from fire and heat sources. Store away from light, and the packaging must be sealed and not in contact with air. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. storeThe area should be equipped with emergency spill response equipment and appropriate containment materials.
Synthesis method
1. It is obtained by hydrolyzing, dehydrating and distilling pentosan-rich agricultural waste, such as corn cobs, cottonseed hulls, rice bran and sugar beet pulp, with dilute acid.
2. Furfural was originally obtained from furfural. Agricultural and sideline products mostly contain polypentoses which undergo hydrolysis to generate furfural. The stems, bark, and shells of many crops contain polypentosides and can therefore be used as raw materials for the production of furfural. During production, raw materials such as corn cobs, cottonseed husks or sugarcane bagasse are treated with sulfuric acid and steam, and then steam distilled, layered, and distilled under reduced pressure to obtain a product with a purity of 99%. The recovery of furfural is related to the raw materials, the type and concentration of the acid and other conditions, and is usually quite different from the theoretical yield. There are two main methods for manufacturing furfural industrially. The pressurization method is suitable for large-scale production. The raw materials and dilute sulfuric acid are cooked under pressure, and the reaction product is taken out with high-pressure or superheated steam. After fractionation, the finished product of furfural is obtained; the normal pressure method is to combine the raw materials with inorganic salts such as salt and Boil with dilute sulfuric acid and steam out furfural at the same time.
3. Crush 100kg corn cobs into small pieces of 0.5~1cm2, add 25kg 90% sulfuric acid and 125kg salt and water to prepare a hydrolyzate. The volume is 2.5 times that of corn cob. After the corn cobs and hydrolyzate are stirred evenly, they are heated to boiling, and then the dilute solution of furfural begins to distill out, and is condensed and collected in a separator. Leave to stand for 1 to 2 hours to separate the aqueous phase and obtain crude furfural. Refined by steam distillation to obtain pure product.
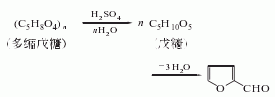
Refining method: Furfural is produced when placed The acidic substances and resins can be removed by washing with water and distilling under reduced pressure. It can also be dried with calcium chloride, anhydrous magnesium sulfate or anhydrous sodium sulfate and then distilled under reduced pressure. The recovery method of used furfural is steam distillation followed by fractionation. Other refining methods include distillation in the presence of 7% sodium carbonate, distillation with 2% sodium carbonate added to the distillate, and finally fractionation under reduced pressure at 800 Pa to obtain the pure product.
Purpose
1. Furfural is a raw material for preparing a variety of drugs and chemical products, such as 2-furancarboxylic acid, furan, and tetrahydrofuran. 1,4-dichlorobutane can be produced from tetrahydrofuran, which is then substituted with a cyano group and then hydrogenated to obtain 1,6-hexanediamine, which is the basic raw material for the synthesis of nylon 66. Furfural can also be used to produce nitrofuracil, furanocrolein, furanoacrylic acid, furfuryl alcohol, etc., which are important raw materials for the synthesis of medicines, pesticides, veterinary drugs, dyes, spices and other products.
2. It is used as an extraction agent to extract unsaturated hydrocarbons from hydrocarbon mixtures, extract butadiene from C4 hydrocarbons, and extract aromatic hydrocarbons from a mixture of aliphatic hydrocarbons and aromatic hydrocarbons. It is also used for the refining of lubricants, natural oils, crude anthracene, etc., the concentration of vitamins A and D, and the solvent of natural resins. In addition, furfural is also used in the preparation of furan resins, electrical insulation materials, varnishes, nitrofuracil, maleic anhydride, tetrahydrofuran, furfuryl alcohol, etc.
3. Determination of cobalt and determination of sulfate. Reagents for the determination of aromatic amines, acetone, alkaloids, vegetable oils and cholesterol. Use as a standard when measuring pentoses and polypentoses. Synthetic resin, refined organic matter, nitrocellulose solvent, dichloroethane extractant.
4. Used as a chromogenic reagent for the determination of carbamates by thin layer chromatography. It is also used as a standard reagent for the determination of pentoses and polypentoses. And used in organic synthesis and as solvent.
5. Used as a solvent, and as an intermediate for the synthesis of fragrances, furfuryl alcohol, and tetrahydrofuran. [27]
extended-reading:https://www.bdmaee.net/n-dimethylcyclohexylamine-2/extended-reading:https://www.newtopchem.com/archives/526extended-reading:https://www.newtopchem.com/archives/43944extended-reading:https://www.bdmaee.net/22-dimorpholinodiethylether-3/extended-reading:https://www.newtopchem.com/archives/category/products/page/78extended-reading:https://www.newtopchem.com/archives/45078extended-reading:https://www.newtopchem.com/archives/44222extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/DBU-octoate–SA102-Niax-A-577.pdfextended-reading:https://www.cyclohexylamine.net/polyurethane-low-odor-catalyst-polyurethane-gel-type-catalyst/extended-reading:https://www.bdmaee.net/nt-cat-pmdeta-catalyst-cas3855-32-1-newtopchem/