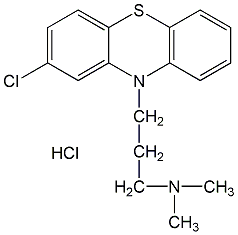
Structural formula
| Business number | 01FJ |
|---|---|
| Molecular formula | C17H20Cl2N2S |
| Molecular weight | 355.34 |
| label |
2-Chloro-10-(3-dimethylaminopropyl)phenothiazine hydrochloride, hibernation spirit, Colagent, Chlorpromazine, aminazine, chlorpromazine, Chlorpromazine hydrochloride, Hibernating phosphorus, C17H19ClN2S·HCl |
Numbering system
CAS number:69-09-0
MDL number:MFCD00012654
EINECS number:200-701-3
RTECS number:200-701-3
BRN number:3779989
PubChem number:24277891
Physical property data
1. Character:White or milky white crystalline powder, hygroscopic, slightly odorous, extremely bitter in taste, easily oxidized in air or Gradates to reddish brown in sunlight
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):179-180℃( 194-196℃ ).
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Unsure
7. Refractive index:Not sure
8. Flash point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): No��determined
11. Vapor pressure (kPa,25ºC): Unsure
12. Saturated vapor pressure (kPa,60ºC): Unsure
13. Heat of combustion (KJ/mol): Unsure
14. Critical temperature (ºC): Unsure
15. Critical pressure (KPa): Unsure
16. Oil and water (octanol/Log value of water) partition coefficient: Uncertain
17. Explosion limit (%,V/V): Unsure
18. Lower explosion limit (%,V/V): Unsure
19. Solubility:Easily soluble in water, ethanol, chloroform, insoluble in ether and benzene
Toxicological data
None
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 31.8
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 339
10. Isotopic origin�Quantity: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine chemical bonds Number of stereocenters: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 2
Properties and stability
None
Storage method
Keep it sealed and protected from light.
Synthesis method
By2-Chlorophenothiazine[92-39-7]Condensation and salt formation. 1.Condensation in reaction Add 2- Chlorophenothiazine and toluene, then add sodium hydroxide, stir and heat to reflux, remove moisture, and in 110℃ dripping1-Chlorine-3-Toluene solution of dimethylaminopropane, the reactant is decompressed to recover toluene to obtain chlorpromazine,C17H19CIN2S,[50-53-3]. 2.Salt Stir and heat chlorpromazine and isopropyl alcohol until 40℃, access dry hydrogen chloride to pH 4-5, and the chlorpromazine hydrochloride is obtained as a salt.
Purpose
has central stabilizing, antiemetic, anticonvulsant, anti- Adrenaline and other effects. Used to treat schizophrenia and mania. Anxiety disorders, mental disorders, nausea, vomiting, etc. It can also be used for artificial hibernation and low-temperature anesthesia.
extended-reading:https://www.bdmaee.net/fascat9201-catalyst-dibutyl-tin-oxide-fascat9201/extended-reading:https://www.newtopchem.com/archives/45168br>extended-reading:https://www.newtopchem.com/archives/40312extended-reading:https://www.bdmaee.net/nt-cat-ea-33-catalyst-cas280-57-9-newtopchem/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/18-Diazabicycloundec-7-ene-CAS-6674-22-2-DBU.pdfextended-reading:https://www.morpholine.org/category/morpholine/other-products/extended-reading:https://www.bdmaee.net/n-butyltintrichloridemin-95/extended-reading:https://www.bdmaee.net/dabco-t-9-catalyst-cas29568-56-9-evonik-germany/extended-reading:https://www.newtopchem.com/archives/category/products/page/41extended-reading:https://www.newtopchem.com/archives/44333
