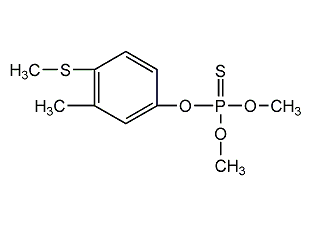
Structural formula
| Business number | 016T |
|---|---|
| Molecular formula | C10H15O3PS2 |
| Molecular weight | 278.33 |
| label |
O,O-dimethyl-O-[3-methyl-4-(methylthio)phenyl]-phosphorothioate, O,O-Dimethyl-O-[3-methyl-4-(methylthio)phenyl]phosphorothioate, Baycid, Lebaycid, Phenthion Tiguvon, Bai Zhi Tu, fenthion, timesex, fenxapine, Organophosphorus pesticides |
Numbering system
CAS number:55-38-9
MDL number:MFCD00055449
EINECS number:200-231-9
RTECS number:TF9625000
BRN number:1974129
PubChem number:24862533
Physical property data
1. Properties: brown liquid. Slightly smell of garlic.
2. Density (g/mL, 25/4℃): 1.250
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 319
5. Boiling point (ºC, normal pressure): 87
6. Boiling point (ºC, 5.2kPa): Undetermined
p>
7. Refractive index: 1.5698
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa , 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15 . Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V ): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in methanol, ethanol, ether, acetone and various Other organic solvents, almost soluble in water (55mg/L)
Toxicological data
Toxic acute exposure of male rats to LD50 is 215 mg/kg, and that of female rats is 24Jmg/kg. The acute transdermal LD50 in rats is 330-SOOmg/kg. The maximum allowable dose in the 60-day feeding test in rats was 10 mg/kg. Feeding dogs at a dose of 50 mg/kg for 1 year had no effect on their body weight and food intake. The LC50 for fish is about 1 mg/L (48h). For bees, parasitoids, aphids, etc.The insect is highly poisonous.
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 72.23
2. Molar volume (cm3/mol): 221.8
3. Isotonic specific volume (90.2K): 586.5
4. Surface tension (dyne/cm): 48.8
5. Polarizability (10-24cm3): 28.63
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 85.1
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 254
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Yi Luo is drunk in A, B, Jia, and Er. Toluene, acetone, chlorinated hydrocarbons, fatty oils and other organic solvents are difficult to dissolve in petroleum ether, and their hydrolysis degree in water is 54 – 56mg/L. Industrial products are brown in color and have a special odor. It is relatively stable to light and heat, and is more stable than methyl parathion when hydrolyzed in acidic or alkaline media. At 100′, in pH value 1.8~5 medium, the hydrolysis half-life is 36h, in pH value 11 medium, the hydrolysis half-life is 95min. Hydrogen or potassium permanganate can oxidize the thioether chain to generate the corresponding subsulfa and sulfa compounds.
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
The intermediate p-methylthio m-cresol is prepared by reacting m-cresol with dimethyl disulfide, and then reacted with O, O-dimethylthiophosphoryl chloride to obtain fenthion.
1. Preparation of p-methylthio m-cresol. Mix m-cresol (or mixed cresol) and dimethyl disulfide at a ratio of 2:1 (weight ratio), add to the reaction pot, stir and cool to 10- 15℃, start adding concentrated sulfuric acid dropwise. Complete the dripping in about 1 hour (the amount is equal to the weight of dimethyl disulfide), and continue the reaction at 10-15°C for 3 hours. Let stand and separate into layers, discarding the waste acid in the lower layer. The organic layer was neutralized with 10% sodium carbonate solution, and then washed with water at pH 7-8. Recover unreacted dimethyl disulfide and cresol through vacuum distillation, and the remaining material is the intermediate p-methylthio-m-cresol.
2. Preparation of fenthion Add 20% sodium hydroxide solution to the reaction pot, stir and cool to below 25°C, add the intermediate prepared above, and then add O, O- evenly at 10-20°C Dimethyl thiophosphoryl chloride should be dripped in about 30-40 minutes. The molar ratio of the three materials is 1.3:1:1.2. Slowly raise the temperature to 60°C and keep it warm for 2 hours. Check the pH changes during the reaction. When the pH is <9, sodium hydroxide solution should be added. After the reaction is completed, the temperature is lowered to 45°C, washed with sodium hydroxide solution, then washed with water, and left to stand for layering. The lower organic layer was removed and dehydrated under reduced pressure, cooled, and filtered to obtain fenthion crude oil with a content of 90% and a yield of 90%.
Purpose
1. Pesticides, insecticides, acaricides. Cholinesterase inhibitors. Organic Synthesis. Fenthion is an organophosphorus insecticide with low toxicity to humans and livestock. It is effective against a variety of pests. It mainly acts as a stomach poison for contact killing. It has a long residual effect. It is not as effective against mites as methyl parathion. It is mainly used for prevention and control. It has good effect on soybean borers, cotton pests, fruit tree pests, vegetable and rice pests, and is used to control mosquitoes, flies, bedbugs, lice, and cockroaches. 2.A broad-spectrum, fast-acting, toxic organophosphorus insecticide that is also effective against mites. It has contact and stomach poisoning effects, strong permeability, certain systemic effects, and long residual effect. It can be used to control rice, cotton, fruit trees, soybeans and other crops to control stem borer, stem borer, rice leafhopper, rice bractworm, rice leafroller, pink bollworm, cotton bollworm, cotton aphid, cabbage caterpillar, and vegetable aphids. , fruit tree borers, scale insects, citrus rust ticks, web bugs, tea poisonous moths, tea green leafhoppers, soybean borers and sanitary pests. For example, to control rice pests, cotton bollworm and pink bollworm, use 50%, EC 11.3~15m Work_/100m2 Spray water; to control cotton spider mites, cotton aphids, vegetable aphids, vegetable aphids and other pests, use 50%, EC 7.5~11 .3mL/100mL/m2 Spray with water; to control cotton spider mites, cotton aphids, cabbage aphids, vegetable aphids and other pests, use 50 “/t, EC 7.5~11 .3mL/100mL/m2 Spray with water; to control large heartworms, use 50%, EC 7.5~11.3mL/100mL/m2, Spray with water, the effect is 80 0/t, or above, or use 3%, Powder, 225~300kg/1002 spray powder; 50 for controlling citrus rust tick and clam weevil, EC Sichuan Yin Liquid spray
extended-reading:https://www.newtopchem.com/archives/44787extended-reading:https://www.newtopchem.com/archives/40434extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Catalyst-8154-NT-CAT8154-polyurethane-catalyst-8154.pdfextended-reading:https://www.bdmaee.net/u-cat-sa-831-catalyst-cas111-34-2-sanyo-japan/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/102-6.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/1-6.jpgextended-reading:https://www.cyclohexylamine.net/monobutylzinntrichlorid-cas-1118-46-3/extended-reading:https://www.bdmaee.net/polycat-17-catalyst-cas110-18-9-evonik-germany/extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/75.jpgextended-reading:https://www.cyclohexylamine.net/dabco-pt305-low-odor-reactive-amine-catalyst-pt305/
